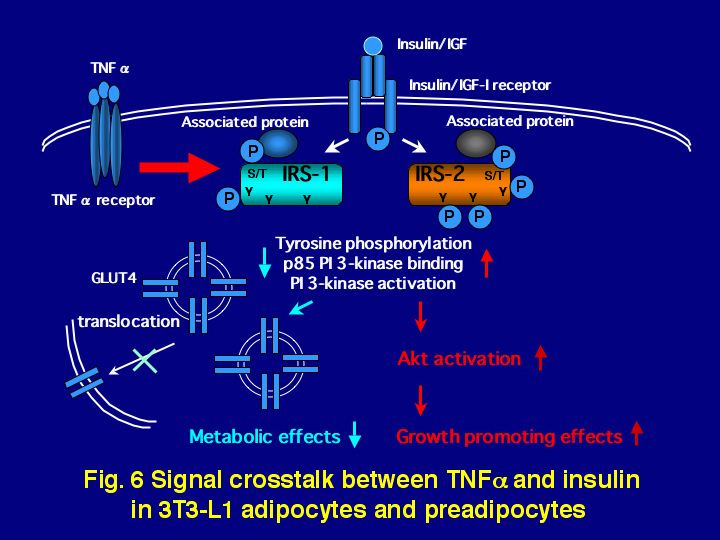
TNF (tumor necrosis factor)- ƒ¿ has been shown to cause insulin resistance in vivo and in vitro. Especially in adipocytes, there are accumulated data indicating that TNF-ƒ¿ inhibits insulin signaling at the early stages. However, the molecular mechanisms of insulin resistance caused by TNF-ƒ¿ are still unclear. As others reported, in 3T3-L1 adipocytes, we confirmed that TNF-ƒ¿ decreased insulin or IGF-I-dependent tyrosine phosphorylation of IRS-1 and further inhibited binding of a p85 PI 3-kinase to IRS-1. Surprisingly, TNF-ƒ¿ increased IRS-2 tyrosine phosphorylation as well as its binding to p85 PI 3-kinase. In our cell-free phosphorylation assay using IGF-I receptor or insulin receptor as a kinase, tyrosine phosphorylation of IRS-1 from cells treated with TNF-ƒ¿ decreased, but IRS-2 tyrosine phosphorylation increased. These results suggested that TNF-a treatment caused changes in IRS-1 and IRS-2 by different mechanisms, resulting in differential phosphorylation by receptor kinases. Recently we found that IRS-1-associated proteins decreased availability of IRS-1 to insulin receptor kinase and we are endeavoring to identify these proteins. Finally, we measured insulin-dependent glucose uptake or IGF-induced growth in 3T3-L1 adipocytes or pre-adipocytes pretreated with TNF-ƒ¿ for 24 h. Our results show that TNF-ƒ¿ significantly depressed glucose uptake induced by insulin, but potentiated cell growth induced by IGF-I, which is correlated with the change of IRS-1 or IRS-2 tyrosine phosphorylation, respectively. These results suggest that IRS-1 and IRS-2 possess different physiological roles in insulin/IGF signal transduction (Fig. 6).