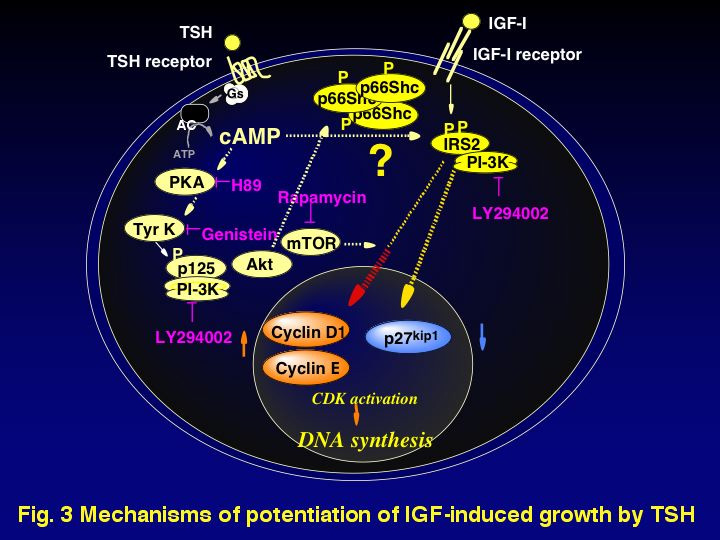
We have shown that in FRTL-5, a thyroid follicular cell line, TSH and IGF-I stimulate cell growth synergistically and TSH pretreatment through activation of cAMP signaling pathway is essential for the potentiation of IGF-I-dependent DNA synthesis. We are utilizing this model to elucidate the mechanisms by which various factors potentiate IGF bioactivities (Fig. 3).
In FRTL-5 cells, cAMP pretreatment caused an increase in tyrosine kinase activity and tyrosine phosphorylation of intracellular proteins such as a 125-kDa protein (p125), which was well correlated with a cAMP-priming effect on potentiation of DNA synthesis induced by IGF-I. We found that the phosphotyrosyl p125 bound to a PI 3-kinase p85 regulatory subunit, and a PI-3 kinase inhibitor blocked the cAMP-priming effect. Recently, we succeeded in identifying p125 (we named it PI3KAP/XB130) and it is a novel protein possessing two pleckstrin homology (PH) domains and a p85 PI 3-kinase recognition motif containing tyrosine residues that may be phosphorylated. On the other hand, we demonstrated that cAMP pretreatment potentiated IRS-2 and Shc tyrosine phosphorylation induced by IGF-I, although pretreatment with cAMP did not affect autophosphorylation of the IGF-I receptor. cAMP pretreatment increased Grb2 binding to IRS-2 and Shc, and MAP kinase activation induced by IGF-I was also enhanced by cAMP stimulus. Furthermore, cAMP pretreatment increased IRS-2 association with a p85 PI 3-kinase, and IGF-I-induced PI-3 kinase activity bound to IRS-2 was enhanced by cAMP pretreatment. Finally, the presence of a MEK inhibitor or a PI 3-kinase during IGF-I treatment abolished the cAMP-dependent augmentation of IGF-I dependent DNA synthesis. These results suggest that cAMP stimulus amplifies the IGF-I signals through cAMP-dependent potentiation of tyrosine phosphorylation of IGF-I receptor substrates. In addition, we found that availability of IRS-2 to the IGF-I receptor has been increased via serine/threonine phosphorylation followed by association of other proteins with the complex. Experiments are in progress to identify intramolecular modulation of IRSs as well as IRS-associated proteins by yeast two-hybrid screening and LC-MS/MS analysis of co-immunoprecipitated proteins with IRS.
Lastly, we are elucidating how cAMP and/or IGF-I stimulus regulate the G1 cyclins- CDKs-inhibitors system, and determining the roles of PI 3-kinase activation by cAMP or IGF-I stimulus in this system. We found that cAMP pretreatment enhanced IGF-I- dependent increases in cyclin D1, due to synergistic increases in mRNA and elevation of translation rates. Furthermore, cAMP pretreatment enhanced protein degradation of CDK inhibitor, p27Kip1 induced by IGF-I. These changes induced an increase in cyclin E leading to marked activation of G1 cyclin-dependent kinases followed by Rb phosphorylation. Our results using a PI 3-kinase inhibitor showed that cAMP-dependent PI 3-kinase activation plays an important role in an increase in cyclin D1 translation. In contrast, IGF-I-dependent PI 3-kinase activation was required for synergistic increase in cyclin D1 mRNA levels and degradation of p27Kip1. We conclude that PI 3-kinase controlled by cAMP or IGF-I stimulus plays different roles for synergistic changes in cyclin D1 and p27Kip1, leading to cAMP-dependent potentiation of CDK activation and DNA synthesis induced by IGF-I. This gcross-talkh mechanism may mediate the synergistic effects of tropic hormones and IGF in endocrine cells.