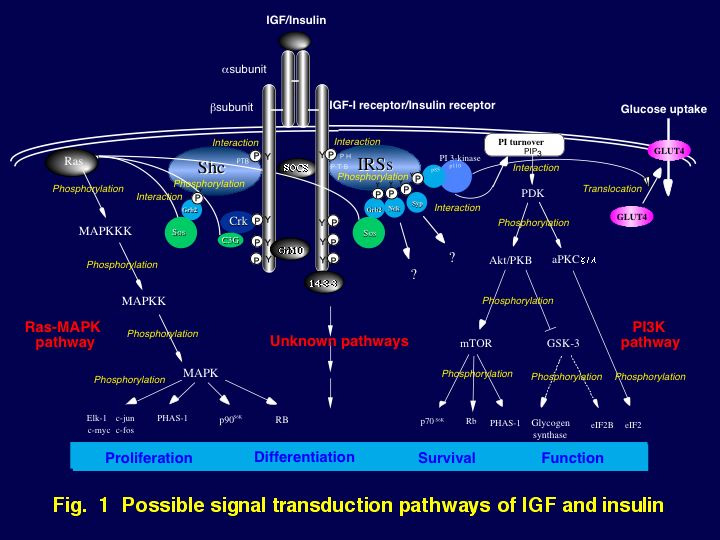
In general, binding of IGF-I or insulin to its specific receptor on a target cell membrane is followed by activation of tyrosine kinase in the ƒÀ-subunit of the the cognate receptor. The activated IGF-I receptor or insulin receptor in turn phosphorylates specific substrates, in particular insulin receptor substrate (IRS)-1, IRS-2, and Src homology collagen (Shc). Recently, it has become clear that phosphorylated tyrosine residues of IRS-1, IRS-2, and Shc are recognized by various signaling molecules that contain a Src-homology region (SH)-2 domain. These include Grb2 and an 85 kDa regulatory subunit of phosphatidylinositol 3-kinase (PI 3-kinase), suggesting that binding of tyrosine phosphorylated substrates to these SH2 domain-containing proteins mediates IGF-I bioactivities. These associations are thought to stimulate the MAP kinase cascade or the PI 3-kinase cascade, well-known signaling pathways, which mediate many significant actions of growth factors (Fig. 1).
Clearly, novel signaling pathways must exist to mediate specific biological activities. In addition, we would like to know how the signaling of IGF-I differs from insulin, because IGF-I and insulin are shown to share common signaling pathways, but IGF-I predominantly mediates long-term action to determine the cell fates and insulin mainly possesses metabolic activity. These questions should be solved in the near future.